- 金属腐蚀的化学原理
- 共23题
2.能证明BF3为平面三角形而不是三角锥形分子的理由是( )
正确答案
解析
A.BF2Cl只有一种结构,可能为三角锥型,不一定为平面三角形,故A错误;
B.BF3中B的价电子结构为2s22p1,价电子对数为: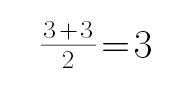
C.BFCl2只有一种结构,可能为三角锥型,不一定为平面三角形,故C错误;
D.三根B-F键键长都为130pm,可能为三角锥型中棱相等,不能说明BF3为平面三角形,故D错误;
考查方向
原子组成与结构
解题思路
根据价电子对数判断其杂化类型,根据形成的杂化轨道判断其空间构型,BF3中B的价电子结构为2s22p1,形成了三条杂化轨道,B的杂化类型为sp2,三个sp2杂化轨道分别与三个F原子的p轨道成键,三根B-F键间键角都为120°,为平面三角形,BFCl2、BF2Cl只有一种结构,三根B-F键键长相等,也可能为三角锥型
易错点
键长相等,也可能为三角锥型
教师点评
本题主要以BF3的分子构型考查了杂化轨道理论的应用,题目难度不大
知识点
Dogs are often【C1】______man’s best friend. For those who are blind, a dog is【C2】______than a friendly company. A dog is a pair of sharp eyes. A【C3】______dog guide gives blind people the【C4】______to move through the world safely and freely. A dog guide is also a best friend. 【C5】______a working team of a dog and a person is not easy. There are no factories making thousands of dog guides a day. There are however, schools all【C6】______the country that train both blind people and the dogs they depend upon. The process of education is long for both the dog and its human partner. A dog begins schooling as puppy. Around age of 8 weeks, the puppy is placed with a faster family. There it【C7】______training and gets used to【C8】______around people. When a dog is about a year old, it goes off to school for a series of tests. These tests help the school【C9】______whether the dog is likely to make a good guide. Dogs that fail the tests are placed in permanent homes. 【C10】______that pass the tests go into a training【C11】______ For the next four-to-six months, the dog learns everything【C12】______needs to become a dog 【C13】______The dog learns to stay focused. It learns to ignore food, smell and other animals. It learns to remain【C14】______by such noises such【C15】______shouting or music from a passing car radio. It learns to【C16】______commands like "right", " left" or " forward ". The dog learns to stop before every curb and stairway and to【C17】______moving objects. In short, good dog guides learn to keep their【C18】______safe while they go about the everyday actions of life. A dog that【C19】______completed training will be【C20】______a master.【C11】
A.test
B.practice
C.play
D.programme
正确答案
D
解析
暂无解析
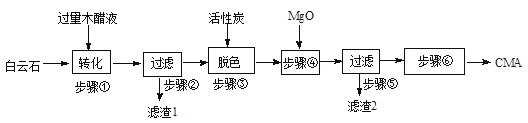
CMA(醋酸钙、醋酸镁固体的混合物)是高速公路的绿色融雪剂。以生物质废液——木醋液(主要成分乙酸,以及少量的甲醇、苯酚、焦油等杂质)及白云石(主要成分MgCO3·CaCO3,含SiO2等杂质)等为原料生产CMA的实验流程如下:
8.步骤①发生的反应离子方程式为 。
9.步骤②所得滤渣1的主要成分为____________(写化学式);步骤②所得滤液常呈褐色,颜色除与木醋液中含有少量的有色的焦油有关外,产生颜色的另一主要原因是____________。
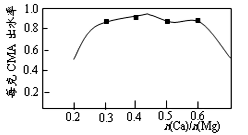
10.已知CMA中钙、镁的物质的量之比与出水率(与融雪效果成正比)关系如右图所示,步骤④的目的除调节n(Ca)∶n(Mg)约为____________(选填:1∶2;3∶7;2∶3)外,另一目的是 。
11.步骤⑥包含的操作有____________、过滤、洗涤及干燥。
12.碳酸镁和碳酸钙与醋酸也可以恰好完全反应得到的混合物制融雪剂,下列有关说法错误的是( )
正确答案
MgCO3·CaCO3+4CH3COOH= Ca2++Mg2++4CH3COO-+2CO2↑+2H2O;
正确答案
SiO2;实验过程中苯酚被空气中氧气氧化最终产生褐色物质;
正确答案
3∶7;除去过量的乙酸;
正确答案
蒸发结晶
正确答案
扫码查看完整答案与解析